FAQs
In general you must have approval before initiating activities with recombinant DNA.
请参阅我们的“调查员职责” Policies page.
通过以下途径提交生物安全表格 VERAS.
如果您对如何填写表格有疑问 Biosafety Form,请与 IBC协调员 or the 生物安全官 (BSO)寻求协助.
While many of the commercially available vectors are exempt, you must file a Biosafety Form 以确定IBC的承保或豁免地位. 所有涉及的工作必须得到IBC的批准.
三年,除非另有说明.
It is the Principal Investigator's responsibility to renew a protocol. 这是通过提交一个协议来完成的 VERAS. The 研究保证处 (ORA) has developed a system whereby in the future every attempt will be made to send a reminder in advance of the three years expiration to investigators to assist them in managing this compliance piece.
Biological toxins are poisonous substances that can be produced by animals, plants, insects, fungi, 和微生物. Biological toxins are not considered infectious because they do not replicate, 然而,少量的它们可能是剧毒的.
Biological toxins are typically handled with biosafety level 2 practices and facilities. Safe lab practices and controls must be implemented before working with biological toxins. Standard operating procedures (SOP) must be implemented in the lab before use. 毒素在处理前必须灭活.
Some biological toxins are considered Select Toxins under the Center for Disease Control and Prevention (CDC) Select Agent Program. 请与 生物安全办公室 if you plan to perform research with CDC选择代理 and Toxins as there may be additional requirements when working with these materials.
These are agents and toxins that have been determined to have dual use potential by the federal government and require registration and approval from the federal government to work with.
View a 美国国家卫生与公众服务部制作的7分钟视频 更多关于什么是“双重用途”的信息.”
See the 选定药剂和毒素的最新清单.
All work with Select Agents and Toxins must be submitted via a Biosafety Form to ibc@sgmtc678.com.
Work that requires Federal oversight must be registered with CDC. 可能需要6个月或更长时间才能获得批准. Contact the 生物安全官 为这个过程提供帮助.
一些人与 Select Agents and Toxins is excluded from federal oversight.
There are permissible toxin amounts which are also excluded from federal oversight.
VERAS FAQs
- 登入VERAS系统(veras.sgmtc678.com/)和你的网络ID和密码.
- Verify My Workspaces 设置为“学习助手”
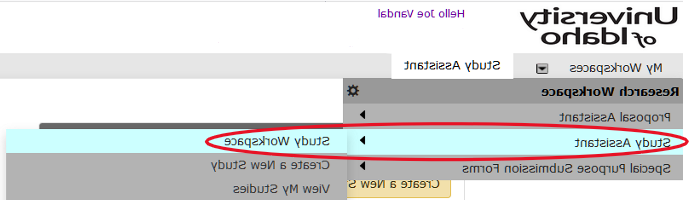
- 选择下面的“查看我的研究” 特色研究操作,或向下滚动至 All Studies,

- 如果你有提交给其他委员会的意见, you may need to select the down arrow to view only IBC protocols.
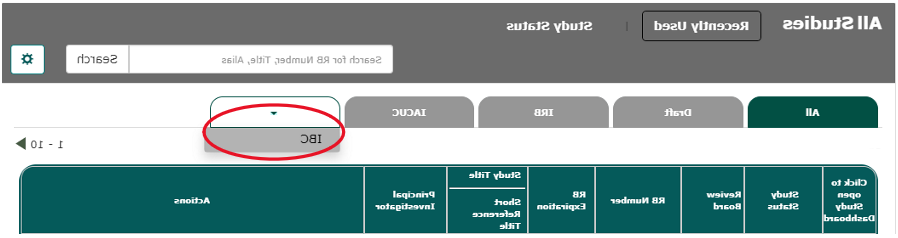
- Login to veras.sgmtc678.com
- Select 查看我的学习 under 特色研究操作,或向下滚动至 All Studies,在要修改的协议行上选择
- 将出现一个弹出窗口,在 IBC 更新、修改 & Closure Forms, select
(开始一个新的提交) IBC -仅限人事变动
- 遵循以下适当的说明:
- 如果要添加Co-PI,请选择
A行旁边的按钮.)额外调查人员.
- 如果要增加研究人员,请选择
,下面绿色突出显示,在下一行B.)研究人员.
- 如果要删除内部人员,请选择
下面以橙色突出显示.
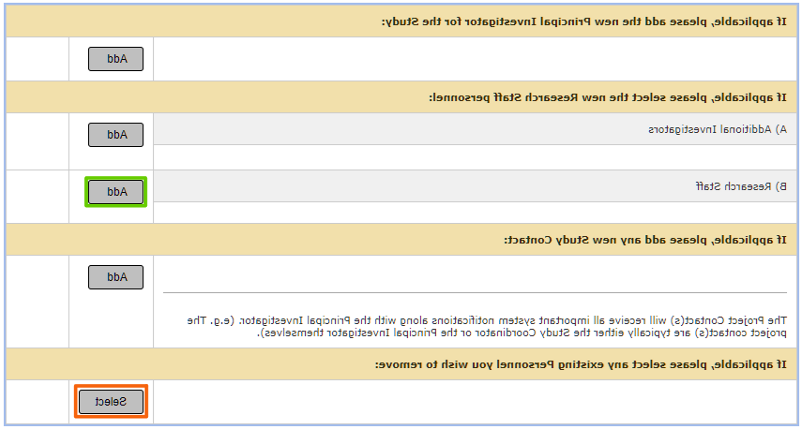
- 如果要添加Co-PI,请选择
Please note, if adding personnel to work with export controlled items (EAR and ITAR第十四类)或指定代理,您必须提交一份 "IBC -仅限人事变动" form and receive IBC approval, 在提交修正案将其纳入章节之前 CDC选择代理 & Toxins or Export Controlled Items (EAR and ITAR第十四类).
学生/人员将需要登录到 VERAS, if they do not already have an account, where they will be prompted to create an account. Once the account is created then you will be able to see them in the system.
Please note:
- If the personnel are not affiliated with the University, they should not need to create an account.
- 在…或与…一起工作的人员 export controlled items (EAR and ITAR第十四类)或指定代理 MUST 被列在协议上.
Yes, personnel other than the PI can fill out forms for protocols they are connected to, they must be listed as a Study Personnel on the study to have access to it VERAS. However, the PI will need to complete a final Signoff and Submit before the submission is submitted to the committee.
The submission author must select “Notify PI to Signoff” for the PI to receive an email notice and a VERAS home screen task
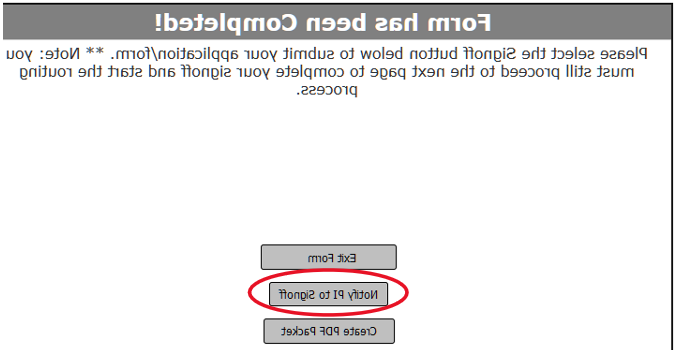 .
.
If the IBC Admin has already started processing the submission, you will need to call or email the IBC and ask them to release the submission so you can make changes. If the IBC has not started processing the submission follow the steps below:
- Login to http://veras.sgmtc678.com/
- Select 查看我的学习 under 特色研究操作,或向下滚动至 All Studies.
- Select the
icon, in the “Click to open” column, for the Protocol you want to edit
- 在屏幕右侧找到 未提交(s) and select “Retract Submission” in the “Process Submission” column.
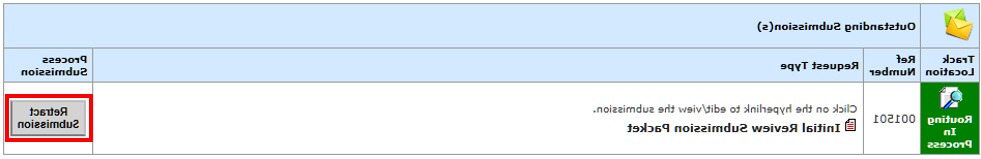
- Login to veras.sgmtc678.com.
- Select 查看我的学习 under 特色研究操作,或向下滚动至 All Studies,在要修改的协议行上选择
- 将出现一个弹出窗口,在 IBC续订、修订 & Closure Forms, select
(开始一个新的提交) IBC -修改表格
- 尽你所能完成表格
- 将出现一个弹出窗口,在 IBC续订、修订 & Closure Forms, select
Notes:
- 在“修订申请”部分 IBC -修改表格,您将选择 点击这里附上申请, then select Add Revision to create a new version of your currently approved protocol. You will update your new protocol version to include the item you are requesting in your amendment.
- 的“提交”部分 IBC -修改表格, attach or revise any additional documents (permits, SOP’s…)
- Please note, if adding personnel to work with export controlled items (EAR and ITAR第十四类)或指定代理,您必须提交一份 IBC -仅限人事变动 表格并获得IBC批准,然后提交 IBC -修改表格 把他们列入"疾控中心特工"名单 & Toxins or Export Controlled Items (EAR and ITAR第十四类)” section.






